STC’s revolutionary and patented U4Lead technology is already working in an industrial plant supplied by STC to the Nigerian company named Green Recycling Industries. It allows the implementation of the desulphurization reaction starting from an amino compound, namely UREA, a very cheap reagent, safe for handling/storing/moving. A comparison between this patented process and the traditional ones will be illustrated in this article together with all its advantages and benefits from an economical, operational and an environmental point of view and, last but not least, its connection with another patented process by STC called LEAD3 for the lead oxide hydrometallurgical regeneration.
THE CURRENT SCENARIO
Everyone knows very well that almost all the components of Used Lead Acid Batteries can be recovered and recycled an unlimited number of times and that more than 60% of the World Lead Production is a result of Secondary Lead Metallurgical processes instead of mining activities.
At present, in the traditional battery recycling plants, pyrometallurgy represents the main approach to lead recycling. The process, well consolidated and almost worldwide adopted, has some drawbacks and presents some issues mainly related to SO2 emissions and formation of slags, that are normally considered hazardous wastes.

Regulations on emissions are becoming progressively stringent. Also costs for transportation and disposal of hazardous wastes resulting from this process are constantly increasing. For this reason, the desulphurization of the lead paste is a viable and consolidated process to reduce the waste generated by the smelting process, to reduce the environmental impact of the smelters, to increase productivity and competitivity of secondary lead producers.
ADVANTAGES OF TRADITIONAL DESULPHURIZATION PROCESS
The traditional lead paste desulphurization process via Sodium Carbonate or Sodium Hydroxide is already incredibly beneficial and some of the most important advantages are illustrated hereafter.
First of all, it enhances the following lead smelting operations. For example, by lowering the SOx emissions in the furnace flue gas by 90% or by reducing the amount of slags produced due to the lower amount of iron addition in the charge for Sulphur sequestration.
The reduced amount of slag produced in the furnace also leads to an increased “room” available for the charge and consequently to a higher productivity. Thanks to the desulphurization, the energy consumption is also drastically reduced due to the lower required operating temperatures in the furnace and reduced process cycle duration thus leading to a remarkable money saving.
The desulphurization process additionally avoids the Sulphur contamination of electrolyte in hydrometallurgical and/or electrolytic processes and it also enhances the quality of the sulphated by-products compared to other specific post-treatments like the abatement via Sodium Bicarbonate.
BASIS OF THE TRADITIONAL DESULPHURIZATION PROCESS
The chemistry of the traditional desulphurization is relatively simple and consists in the reaction between an alkali chemical (soda ash or caustic soda) and the lead sulphate that is converted into Lead carbonate or lead hydroxide.
The sulphuric acid contained in the electrolyte is also transformed into sodium sulphate according to the following reactions:
PbSO4+Na2CO3-à PbCO3 + Na2SO4
PbSO4+2NaOHàPb(OH)2+ Na2SO4
H2SO4+Na2CO3 à Na2SO4+H20+ CO2
H2SO4+NaOH à Na2SO4+H20
The sodium sulphate solution obtained as by product from the reaction, after a purification step necessary to remove the contamination from heavy metals, can be crystallized to produce pure sodium sulphate that can be sold to detergent, glass, textile and paper industries.
The equipment required for the desulphurization process consists of stirred reactors, pumps, chemical dosing systems, filter press.
A conceptual flow diagram is shown in the picture below, where the paste is desulphurised simultaneously with the sulphuric acid contained in the electrolyte.

However, the traditional process via soda ash/caustic soda has its own limits:
The demand for sodium sulphate, one of the main filler components used in washing powder production, with the advent of liquid detergents, has become weaker and consequently the sale price has slightly decreased although still presenting positive revenues. Especially when using sodium carbonate with chloride content, there is also a risk of equipment corrosion and this imposes the use of special construction materials like AISI 904L or DUPLEX for heat exchangers and crystallizers. Eventually, the cost of the chemicals sometimes makes the overall process slightly more expensive considering that Sodium Hydroxide is a by-product of the chloro-alkali process and with the reduction of chlorine uses, the cost of caustic soda in certain countries has significantly increased.
STC IMPROVEMENTS THROUGH U4Lead PROCESS
STC has developed and patented an innovative desulphurization process in order to overcome the problems of the chemicals cost/quality ratio and the relatively poor market for sodium sulphate, ensuring at the same time all the advantages of the desulphurization process.
The U4Lead process by STC, uses an amino compound, namely Urea, as chemical for the desulphurization of paste and electrolyte neutralization.
The simplified reaction can be summarized as follows:
CO(NH2)2 + PbSO4+H2O à PbCO3 + (NH4)2SO4
CO(NH2)2 + H2SO4 + H2O à (NH4)2SO4+CO2
The company provides either full crystallization system to produce pure ammonium sulphate crystals or just an evaporation system in order to produce a concentrated solution of ammonium sulphate “ready-to-use”.
The use of an amino compound as a replacement of the soda ash/caustic soda, leads to a higher degree of conversion of lead sulphate (above 97%) and a lower residual Sulphur (below 0.3%). It also guarantees the further reduction of iron addition, slag production and natural gas/oxygen consumption.
In this case, the reaction by-product is the Ammonium Sulphate, a well-known and valuable fertilizer also obtainable as aqueous solution for fertigation, salable at higher price compared to sodium sulphate. No special material is required for the evaporation or crystallization operations of the by-product solution, due to the absence of chlorides from reactants and there is finally no need to employ dangerous chemicals like Sodium Sulphide for heavy metals abatement from the by-product solution.
WHY U4Lead – THE FUNNY STORY
The need to develop an alternative desulphurization process to the ones already available on the market arose some years ago in STC. The company wanted to propose the most up-to-date and efficient lead paste desulphurization process to one of its Clients and of course the one via Ammonium Carbonate was chosen.
Although there is a huge literature about the desulphurization process via ammonium carbonate, there are no existing operating plants on industrial scale employing ammonium carbonate to carry out the desulphurization reaction.
The main disadvantages of using ammonium carbonate as chemical are the higher price of the chemical and the environmental consequences, pollution and safety issues related to production, transport, storage and handling of this compound. Due to the ammonium carbonate high volatility and subsequent ammonia released toxicity, procurement and transportation may become difficult specially because the production of ammonium carbonate is only concentrated in few countries (mainly China and India).
The possible onsite self-production of ammonium carbonate starting from Ammonia and CO2 also has some drawbacks like high cost and safety problems related to ammonia transportation and storage.
Funnily enough, STC ended up realizing immediately that due to the above-mentioned difficulties, it was necessary to look for other valid options.
For this reason, thanks to the great R&D team and the incredible support and wide knowledge of Prof. Renato Guerriero, President of STC at that time, the company pushed itself beyond the limits. STC actually discovered that it was possible to implement an even more efficient desulphurization process, able to overcome the problems of the chemical cost/quality ratio, while ensuring at the same time all the advantages of the most recent desulphurization process.
The U4Lead process by STC, protected by National and International (PCT) patent, is based on the use of UREA as chemical for the desulphurization of paste and electrolyte neutralization. It is a very cheap amino compound, easy to find, to treat, to handle, chemically stable as not subject to sublimation and odor-free.
Its schematic is represented below: to be noted that the desulphurized paste can be either treated in traditional smelters or processed in the LEAD3 system to directly produce ultrapure nanostructured lead oxides thus obtaining one of the key raw materials for battery industry.

U4Lead: TECHNICAL SPECIFICATIONS
Regarding the U4Lead operating conditions, urea is first of all converted through a specific STC skid-mounted package named UREA CONVERTER, in order to transform it into ammonium carbonate.
The urea specific consumption for paste desulphurization is ~75 kilograms/tonULAB, it requires 15 kWh/tonULAB of electric power and 90 kWh/tonULAB (~135 kilogramsSteam(6 bar) / tonULAB) as thermal power.
As by product, 145 kilograms /tonULAB of pure Ammonium Sulphate crystals is obtained: this chemical has a very good market and can be sold, also in liquid form, as fertilizer for agricultural applications.
THE INDUSTRIAL CASE
Finally, the possibility to desulphurize the lead paste via UREA has been verified for the first time in the World on industrial scale: after the first initial tuning at the industrial plant, U4Lead process confirmed the excellent results already obtained in the lab scale testing and it can be easily implemented and integrated into existing plants. The process is based on the application of the Urea Conversion System, illustrated in the pictures below:



U4Lead is a subproject of a larger project for a new complete battery recycling plant entirely supplied by STC to its Nigerian client Green Recycling Industries Ltd. It also includes some other innovative solutions like the metallic lead desulphurization with urea, the paste pelletization and premixing with fluxants, the use of a special Tilting rotary furnace operating at low temperature for the melting of desulphurized metallics, with direct discharge of the lead in the refining kettles.


AMMONIUM SULPHATE: A PRECIOUS BY-PRODUCT
As briefly said before, another incredible advantage of the U4Lead process is its by-product: the ammonium sulphate, in fact, has a very low affinity with Lead, Nichel, Arsenic and other heavy metals and it is therefore easily recovered, crystallized to produce pure crystals and sold as fertilizer. The lead and other heavy metals content in the obtained ammonium sulphate is much lower than the limit indicated by the EU Regulation 2019/1009 about the market of EU fertilising products.

To avoid the additional step of crystallization, it is also possible to commercialize the ammonium sulphate solution directly obtained after the reaction in liquid form to use it for fertigation activities just using a simple and very cost-effective vacuum evaporation system.
Ammonium sulphate coming from lead production is not a complete novelty as this product has already been produced and commercialized by some lead producers around the World (i.e. East Penn and Teck Metals): the ammonium sulphate by-product resulting from their fumes post-desulphurization is regularly sold on the North American market. However, as a consequence, they cannot take advantage of all the above-listed benefits of the pre-desulphurization of the paste.
COMPARISON BETWEEN THE DIFFERENT DESULPHURIZATION OPTIONS
The three desulphurization design figures are compared in this table:
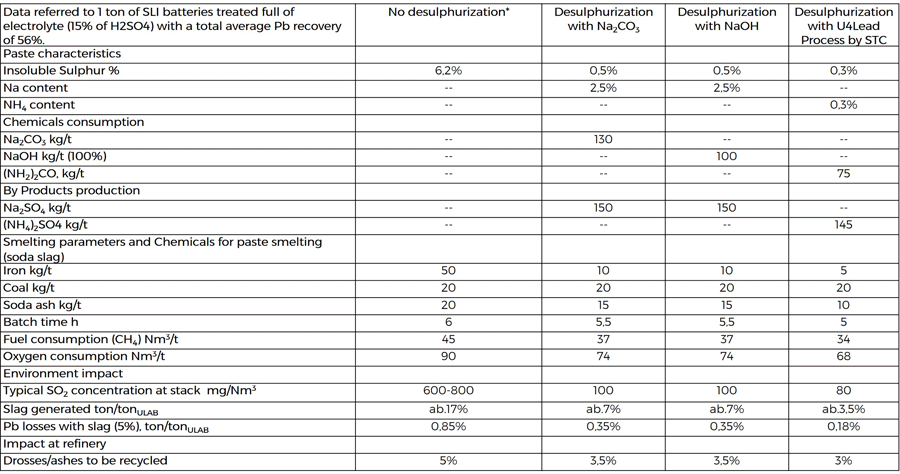
*To be noted that when no desulphurization process is adopted, the recycling plant needs a dedicated section for the neutralization of the sulphuric acid contained in the electrolyte that here has not been considered. In the desulphurization cases, the electrolyte treatment is integral part of the process and is neutralized together with the paste in the same equipment.
As shown, all the analyzed data are improved when U4Lead by STC is adopted, from chemical consumptions to energy reduction and environmental performances.
From U4Lead to Lead3
The U4Lead desulphurization process may also be considered the preliminary step of another interesting and efficient STC patented process which, especially if linked to the U4lead process, can really revolutionize the whole battery recycling procedure.
The Lead3 hydrometallurgical process for the direct production of pure nanostructured lead oxide from paste is based on a lead paste treatment, where the drying temperature is higher than the Lead Carbonate decomposition temperature. This leads to the formation of Lead Oxide, a compound that is easily leached in the following step and it is possible to reprecipitate high purity lead carbonate. The following heat treatment leads to the production of pure lead oxides of different types: litharge, massicot or minium which might be used both for the production of active material for batteries and for any other use this pure compound is thought for (ceramic, glass and rubber industries, etc.). This alternative and completely eco-friendly process leads to the elimination of pyrometallurgy from the lead recycling process unless for the portion related to the metallic lead which is anyway a simple and less energy demanding process specially when previously subject to desulphurization.
Nowadays, most part of the produced lead is used for battery production and that’s why Lead3 is so crucial. The oxide regeneration process allows the elimination of THREE STEPS:
- The conversion of lead paste into pure lead
- The production of lead ingots and/or lead cyclinders
- The use of Melting Barton’s pot or ball mills for the final conversion of Lead into Lead Oxide.
STC has produced an experimental batch of about 1000 starter batteries with the proposed hydro-metallurgical Lead Oxide Regeneration technology and several tests carried out by independent electrochemical laboratories confirmed that the obtained batteries containing the regenerated nanostructured oxides have a greater capacity and a longer life than those produced with traditional processes.

Some alternative hydrometallurgical technologies are currently under evaluation in this sector but their efficiency and cost-effectiveness must still be tested and demonstrated due to their very high energy consumptions. The Lead3 technology, along with the U4Lead process and all STC solutions, is designed and constructed in compliance with the Circular Economy principles and aims at remarkable economic saving, a virtually non-existent environmental impact and the possibility to supply battery producers with pure lead oxides that are ready for the production of new batteries with better characteristics than the traditional ones.
The process is also a valid alternative for the producers of lead oxides: starting from paste instead of pure lead ingots allows a dramatic reduction of the final cost of the produced lead oxides and the increase of the profitability.
CONCLUSIONS
According to STC, the vision about the future of the battery recycling process lies in two principal options:
- U4Lead Desulphurization and smelting of lead paste in traditional rotary furnaces leading to better environmental performances;
- U4Lead Desulphurization followed by Lead3 Oxide Regenerat
STC is able to provide all kinds of desulphurization systems described in this article, using all the chemicals available on local market, coupled with different kinds of evaporation and crystallization systems including direct steam, cold and hot crystallizers, Mechanical Vapour Recompression, Heat Pump.
Pilot plants for testing are also available to demonstrate the performances.
STC Customer Service is available to evaluate how to implement the process into existing facility including the transformation of traditional desulphurization into U4Lead process.